
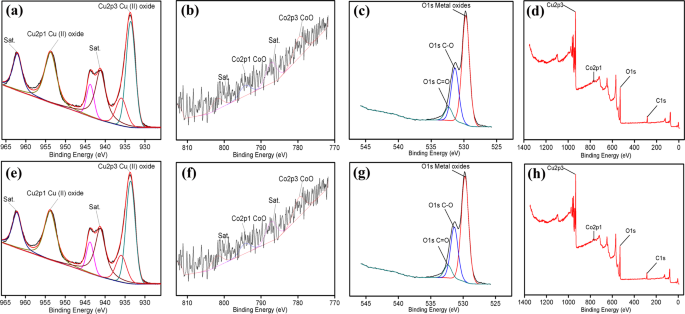
Li L, Zhang MY, Zhang XT, Zhang ZG (2017) New Ti 3C 2 aerogel as promising negative electrode materials for asymmetric supercapacitors. He WD, Wang CG, Li HQ, Deng XL, Xu XJ, Zhai TY (2017) Ultrathin and porous Ni 3S 2/CoNi 2S 4 3D-network structure for superhigh energy density asymmetric supercapacitors. More significantly, Mo-CuO-2 is a promising material candidate for practical energy storage applications. In addition, an asymmetric supercapacitor device was assembled using Mo-CuO-2 and activated carbon as a positive electrode and a negative electrode, which exhibited a remarkable energy density of 36 Wh kg −1 at 810 W kg −1 and an excellent cycle life with 81% capacitance retention for over 5000 cycles. Different doping concentrations of Mo were discussed, and the as-prepared 3 at.% Mo-doped CuO (Mo-CuO-2) exhibited the best electrical conductivity and the highest specific capacitance of 1392 F g −1 at 2 A g −1. Herein, Mo-doped CuO nanosheets on Ni foams were obtained by combining a simple hydrothermal process and calcination treatment. Doping appropriate transitional metal ions into host materials is an effective method to modulate the electronic structure and improve the conductivity, furthermore, enhancing the energy storage capacity. However, their poor electronic conductivity restricts the practical application.
Hydrogen‐reduced CuO and Cu2O were both reoxidized on vacuum annealing, demonstrating the diffusion of lattice oxygen to the surface.Copper oxide (CuO) electrodes have outstanding potentials for supercapacitors in virtue of their low cost, environment friendly, especially, and the high theoretical specific capacitance (1800 F g −1). In the case of CuO, the extent of reduction varies with the thermal history of the sample, with prolonged vacuum annealing producing a more reducible surface. Both CuO and Cu2O are reduced to metal at the surface by heating in 1×10‐4 mbar hydrogen at 400 K. Both bulk Cu2O and the thick Cu2O film generated from vacuum‐annealed CuO were oxidized to CuO by heating at 800 K in 1×10‐4 mbar O2, the original surface being regenerated with vacuum annealing at the same temperature. Prolonged annealing of CuO results in the formation of a thick layer of Cu2O at the surface whilst vacuum annealing of Cu2O produces a thin (possibly one monolayer) film of Cu metal. Using XPS and x‐ray‐excited Auger electron spectroscopy (XAES), we have studied the variation in surface composition of CuO and Cu2O with a variety of high‐vacuum treatments, including vacuum annealing, oxidation and hydrogen reduction. Surface Oxidation and Reduction of CuO and Cu 2 O Studied Using XPS and XAES Surface Oxidation and Reduction of CuO and Cu 2 O Studied Using XPS and XAES


 0 kommentar(er)
0 kommentar(er)
